What You Need to Know About the Water You Use for Watering
Many growers do not realize how critical a role the chemical composition of the water they use to irrigate their plants plays. Alkalinity, pH and the content of dissolved salts are important aspects that determine whether the water is suitable for cultivating plants. In this article we will introduce you to several facts about water that every grower should know to achieve optimal results in their garden.
Whether you grow in hydroponics or in growing media, water is one of the basic resources your plants need. Few growers, however, know that the chemical composition of the water largely affects how suitable it is for cultivation. The main characteristics of water in this context are alkalinity, pH and EC.
Alkalinity expresses the water's ability to resist acidification and is influenced by the concentration of carbonic acid (H2CO3) and bicarbonates (HCO3-). Other compounds such as hydroxides, sulfides, phosphates, silicates or borates also affect alkalinity, but they are usually present in minimal amounts. The higher the water's alkalinity, the less its pH is affected by acidic substances, such as water-soluble fertilizers with a high content of ammonium or urea. Conversely, the pH of water with low or zero alkalinity will drop sharply after adding fertilizers, because it lacks enough buffering capacity to neutralize acids. You can determine water alkalinity from a laboratory analysis, and it is usually expressed as an amount of CaCO₃ or Ca(HCO₃)₂.
Alkalinity above 150 ppm CaCO₃ is considered high and such water will raise the substrate pH. If the water's alkalinity is not too high and ranges between 150–250 ppm, this can be addressed by using a fertilizer that produces an acidic reaction in the substrate. Such fertilizers contain more than 20% of the total nitrogen in the form of ammonium (NH₄+) or urea (the greater the proportion, the more acidic the reaction). In contrast, fertilizers that contain nitrogen only in the form of nitrate produce a neutral reaction.
- Water with alkalinity of 60–150 ppm is considered ideal. Combine it with fertilizers that contain 20–30% of the total nitrogen as NH4+/urea.
- Water with alkalinity below 60 ppm has low buffering capacity. Use fertilizers that contain most of the nitrogen in the form of nitrates (more than 90%) and only a small amount of ammonium or urea.
- If the water's alkalinity is extremely high (above 250 ppm), it is necessary to first lower it by adding acid to approximately 100 ppm and then use fertilizers with a low content of ammonium and urea.
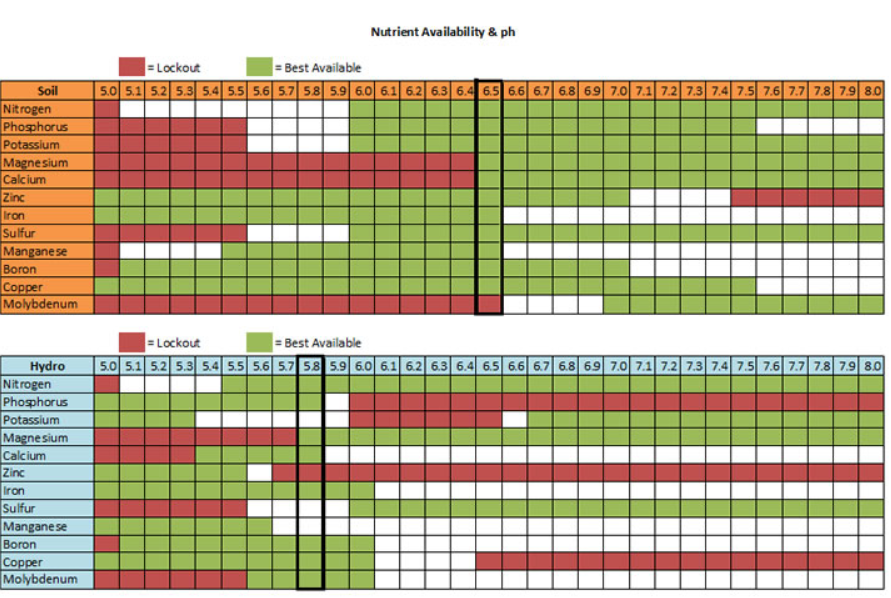
Water pH expresses the concentration of hydrogen ions (H+) and hydroxide ions (OH-) and is an indicator of a solution's acidity or alkalinity. pH is measured on a scale from 0 to 14, where values below 7 indicate an acidic solution, above 7 indicate an alkaline solution, and pH 7 is neutral. The ideal pH range of water intended for irrigation is between 5 and 7, because it is close to the optimal pH for growing most plant species (5.8–6.2). pH is important because acidity affects nutrient availability to plants and extreme values cause nutritional problems.
- If you grow in organic substrates (soil, coco, peat), regularly monitor the substrate pH and keep it in the range 5.5 to 6.5.
- If you grow hydroponically, monitor the nutrient solution pH and keep it constantly in the range pH 5.6 to 6.4.
You can adjust water pH by adding acid or base. To lower pH, nitric, phosphoric, sulfuric or citric acid is most commonly used; citric acid is the only organic option and is therefore safer to handle. To raise pH, products containing soluble magnesium or calcium are used. When adjusting water pH be patient, add acid or base gradually and mix and measure the solution thoroughly each time. You can use litmus paper to measure pH, but if you are serious about growing, it's worth investing in a digital pH meter.
EC stands for electrical conductivity, which indicates how well a solution conducts electric current. In the context of plant cultivation, EC is used to assess the concentration of dissolved salts (ions) in the water or nutrient solution that plants use. These dissolved salts contain mineral elements and nutrients such as nitrogen, phosphorus, potassium, calcium, magnesium and others. Digital EC meters or combined EC and pH meters are used to measure EC.
The lower the EC of the water intended for irrigation, the better, especially if you use fertilizers containing mineral salts. Adding fertilizers increases the water's EC, and too high a salt concentration will burn plant roots, which shows up as burned leaf tips (overfeeding). Rainwater, distilled water or water filtered by reverse osmosis contains no dissolved salts and has an EC of 0 mS/cm. Tap water always contains dissolved salts and its EC depends on the specific region.
- If the EC of the water intended for irrigation is higher than 1 mS/cm (before adding fertilizers) it must first be filtered by reverse osmosis or mixed with distilled water in such a ratio that the EC value drops below 0.5 mS/cm.
- Water with EC 0.5–1 mS/cm (before adding fertilizers) can be used for irrigating plants and for hydroponic systems, but over time salts may accumulate in the substrate and it will need to be flushed with clean water more frequently.
Want to read more about modern plant cultivation methods? Visit our blog.
